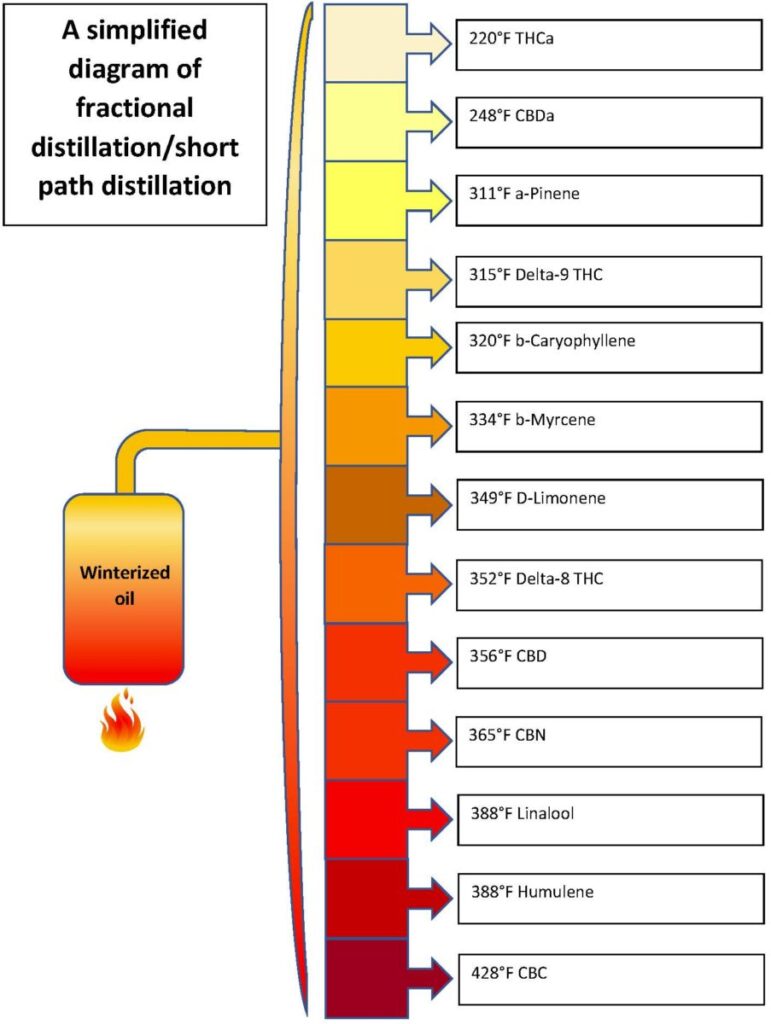
Once you remove the cellulose and water from a plant, not much is left. Do it with a cannabis plant and you are left with an oil that is 50% or more THC. You can even purify it further, given the right equipment each of the several cannabinoids can be extracted in almost pure form called distillates. Converting the plant matter to an oil starts by introducing a solvent, there are several that do the trick. The first oils were called RSO (Rick Simpson Oil) and the extraction used isopropyl alcohol. This type of alcohol is poisonous and the procedure involved careful methods to separate the solvent from the oil. Today we can get the same result using ethyl alcohol (AKA grain alcohol) which is not poisonous (it’s the stuff in whisky). Some people use the term FECO (Full Extract Cannabis Oil) to differentiate it from RSO, but the two are essentially identical with slight variations in the process used. Distillates require fractional distillation equipment and solvents not available to home users. Each of the cannabinoids is extracted from the oil at a different temperature.
Getting The Dosage Right
I make my oil in small batches, using only an ounce or two of buds. The exact amount I use is dependent on the number of portions the recipe I am working on will make. I normally use 1/2 gram of bud per portion. For example, I have a fudge recipe that makes 36 pieces of fudge so I start with 18 grams of bud to make the oil I will need. On average, one gram of marijuana contains about 10% THC, so 1 gram contains about 100 mg of THC. That means each portion will end up with about 50 mg of THC.
The Process
I use a four step process to create my oil:
- Decarboxilation- this step converts the non-psychoactive THCA in the bud to THC.
- Alcohol Bath- this step dissolves the THC into solution so that it can be separated from the cellulose in the next step.
- Filtration- this step removes any remaining solids from the solution.
- Alcohol Extraction- the step removes the alcohol leaving the extracted cannabinoids and terpenes
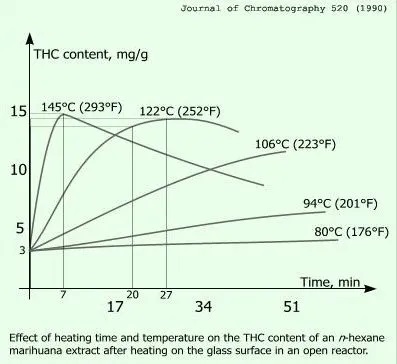
The decarboxilation process was initially described in the Journal of Chromatography in 1990. The graph to the right was taken from that article. It shows how different temperature profiles effected the conversion under laboratory conditions. Notice the characteristics of the curve for 252ºF, it has a relative flat peak spanning about 20 minutes. In practice, it takes a while to get something to the desired temperature, the larger the batch size, the longer it will take (same as boiling a pot of water). When I do decarboxilation, I break the weed into dime sized pieces, then spread them evenly in a baking sheet and cover with foil. Put a piece of parchment under the bud to catch any tricomes that may fall off. I bake for 30 minutes at 250ºF.
IMPORTANT: Alcohol is very flammable use extreme caution in the following steps!
I wrap the decarbed weed in a piece of silk screen material to make a “tea bag”. This will help simplify the filtration in the next step. I place the tea bag in a mason jar and add enough grain alcohol to cover. Put a cover on and give it a good shake. To speed things up, I put the jar in an instant pot with about a half inch of vegetable oil in the bottom to help with heat transfer. Make sure that the cover on the mason jar is very loose so the jar doesn’t break from the pressure caused by heating. I set the pot on warm at 158ºF for one hour. Things dissolve quicker when the solution is heated but you don’t want to heat too much or the alcohol will start to boil.
Filtration is fairly straight forward, I put a coffee filter in a funnel and pour the solution through. I take my tea bag and press it in a juice squeezer to get the liquid out, then I re hydrate it again with some fresh alcohol and squeeze that out too, this second pressing is quite effective. Filter the squeezed liquid and add it to your batch. The weed can now be discarded. If my end product will be translucent like gummies, I will do a second filtration using a syringe filter.
At this point some people put the solution in deep freeze which will cause any plant waxes to precipitate out. I don’t feel this is necessary for the things I make.
There are two ways to separate the alcohol from the canna oil- evaporation or distillation. I use the distillation method, it is faster but lots of caution is required. The boiling point of ethanol is 173.1ºF as your solution approaches this, it will start to boil and vaporize the alcohol. You should never apply heat directly, I always use a hot water bath and monitor it’s temperature very close. In my setup, I have found maintaining a water temperature at 185ºF will put the alcohol solution at the right temperature. Here is a short video showing one of my early attempts. I have since abandoned the mason jar I used as the containment vessel in favor of something more sturdy, but as you can see in the video it works quite well. The great thing about the distillation method is you reclaim the alcohol and can use it over and over. I normally reclaim about 80% of the alcohol, a real cost saver.


Once distillation is complete, you will be left with about a spoonful of oil for every ounce of weed. It is really strong stuff. At this point I mix one of the ingredients from my recipe with the oil. For example, my fudge recipe calls for one stick of butter which mixes easily with the oil. I use corn syrup in my gummy recipe which mixes easily. When I make capsules, I use raw coconut oil because it is solid at room temp and won’t leak. Oil is so versatile!

